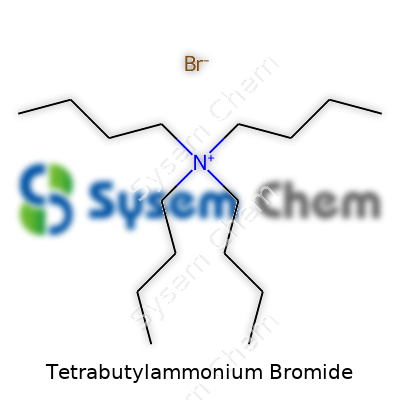
Tetrabutylammonium Bromide: An In-Depth Exploration
Historical Development
Tetrabutylammonium bromide came into regular use during the expansion of organic synthesis in the mid-20th century. Research groups began searching for better ways to drive phase transfer catalysis in both lab and industrial processes. Early scientists found that quaternary ammonium salts filled a gap by easing interactions between organic and aqueous layers. They started using these salts for smoother alkylations and nucleophilic substitutions. Chemical suppliers picked up on this rapidly, as industry demand grew for greener, less hazardous alternatives to traditional strong inorganic bases and solvents. Tetrabutylammonium bromide delivered a simple formula and practical functionality, meeting new regulatory requirements and safety standards as chemical manufacturing changed direction over the decades.
Product Overview
The compound, commonly shortened to TBAB, appears as a white crystalline powder. It gets classified with quaternary ammonium salts and is widely available from chemical suppliers in grades fit for analytical, synthetic, and even pharmaceutical workflows. Users value this salt for its ease of handling, low odor, and high solubility in both water and many organic solvents. TBAB often serves as a phase-transfer catalyst, an application that has solidified its reputation in synthesis. Users can buy it in different purity ratings, from basic technical grade up past high-purity variants for precision-heavy labs. Reliable packaging and straightforward safety data offer clarity for professionals and advanced hobbyists navigating hazardous chemicals.
Physical & Chemical Properties
TBAB forms stable, colorless, odorless crystals with a melting point around 103–106°C. The molecule weighs 322.37 g/mol, and its chemical formula stands as C16H36BrN. These crystals dissolve easily in water and in several organic solvents, including alcohols, acetonitrile, and acetone. TBAB holds up well under normal environmental conditions and does not break down or oxidize quickly. Water solubility comes in at about 650 g/L at 25°C, allowing it to function effectively as a phase-transfer agent and ion-pairing agent. Most users store TBAB at room temperature, away from moisture.
Technical Specifications & Labeling
Manufacturers assign batch numbers and purity specifications when labeling TBAB packages. The most common purity levels are above 98%, which suits most laboratory and industrial synthesis protocols. Labels spell out hazards and proper handling rules per current GHS guidelines. Precise technical data sheets inform end-users on solubility, melting point, and storage life. The salt stays shelf-stable for years under airtight conditions. Reliable suppliers check each batch for residual solvents and moisture, since these can compromise sensitive reactions or product shelf life. Regulatory information on the packaging aligns with REACH, TSCA, and other global chemical use laws, providing transparency.
Preparation Method
Synthesizing TBAB relies on treating tetrabutylammonium hydroxide with hydrobromic acid. Chemists carry out the process in aqueous medium at controlled temperatures, monitoring the reaction for neutralization and absence of free hydroxide. Filtration and washing yield nearly pure salt, which gets dried under vacuum to drive off any trapped water. Labs and industrial plants rely on a process designed for efficiency and minimal byproduct formation. High-purity grades require additional recrystallization, stripping away traces of coloration or impurities that could interfere downstream. Most modern suppliers run small-to-large batch operations, allowing them to meet both specialized and bulk demand.
Chemical Reactions & Modifications
TBAB earns its keep in the lab as a phase-transfer catalyst. It ferries hydrophilic ions into organic media, letting nucleophilic or basic reagents reach substrates hidden away in less polar solvents. As a bromide salt, TBAB readily swaps its bromide for other anions, building custom salts for research and development. During epoxidations, alkylations, and Williamson ether syntheses, it keeps reactions moving at a brisk rate. In practical work, TBAB sometimes acts as a support for ionic liquids or helps create stable emulsions. Chemists tweak its structure by replacing the bromide or changing the alkyl groups on nitrogen, pushing the salt into new roles like anion exchange resin or ionic liquid precursor.
Synonyms & Product Names
Chemicals with more than one use often pick up a string of aliases. TBAB answers to synonyms such as tetrabutylazanium bromide, TBA bromide, or N,N,N-tributyl-1-butanaminium bromide. Distributors may use product numbers like 1643-19-2 to track it in catalogs. The abbreviation "TBAB" signals its role in scholarly papers, technical specs, and online forums. Local nomenclature in various languages often mirrors the compound’s main structure: a quaternary ammonium core, four butyl chains, and a bromide counter-ion. No matter the packaging, those familiar with synthetic chemistry recognize it as a workhorse for countless protocols.
Safety & Operational Standards
Chemists and technicians treat TBAB with the same precaution as all laboratory salts. It causes mild irritation to the skin, eyes, and respiratory tract. Handling aligns with standard chemical hygiene: use of nitrile gloves, goggles, and adequate ventilation helps shut down exposure risks. TBAB does not ignite easily, but storing it away from strong oxidizers prevents any rare mishaps. SDS documentation from every supplier breaks down the product’s reactivity, toxicity, and emergency measures. Waste TBAB, especially from phase-transfer catalysis or industrial streams, must go through approved chemical disposal. Training for proper weighing, mixing, and spill response gives operators confidence in routine handling. Global chemical safety standards—adopted across Europe, the USA, and Asia—inform every step from delivery through final disposal.
Application Area
TBAB’s profile makes it a reliable choice across chemical research and commercial synthesis. In the pharmaceutical sector, it provides the muscle behind alkylations, benzylations, and other key transformations. Material scientists use TBAB in creating new polymers and specialty coatings. TBAB's ability to stabilize nanoparticles opens another set of opportunities in electronics, sensors, and energy storage devices. Water treatment teams tap TBAB for selectivity in ion exchange and extraction processes. Students run undergraduate experiments with it, learning crucial concepts in phase-transfer catalysis. Environmental chemists look for TBAB in wastewater analysis, especially after manufacturing processes. Energy researchers probe its uses as an electrolyte in advanced batteries and electrochemical cells, hunting better ways to move ions around for greener tech.
Research & Development
Groups working at the cutting edge have not finished testing TBAB’s limits. Every year, dozens of papers describe new uses in organic synthesis, from novel catalytic cycles to asymmetric transformations. Teams in electrochemistry fiddle with TBAB’s ratios, hoping to unlock higher yields in batteries or supercapacitors. Some look at incorporating TBAB into renewable energy generation. Analytical chemists optimize chromatography and mass spectrometry protocols with the salt, owing to its mix of charge and solubility properties. There is activity in polymer science, investigating how tetrabutylammonium-based salts alter the surface and structural features of plastics. Graduate students turn to TBAB to push classic reactions faster, cleaner, and colder—saving time and slashing waste. Green chemistry initiatives focus on TBAB as a swap-in for harsher, legacy catalysts, eyeing both health and environmental gains.
Toxicity Research
Toxicology data for TBAB steers clear of alarm, but wise handling wins the day. Standard testing points to low acute toxicity when ingested or inhaled at lab-scale doses, but larger-scale exposures tell a more complicated story. Eyes, skin, and lungs remain sensitive, so proper PPE sees widespread use in every serious lab. Chronic exposure studies stretch thin, and so regulatory bodies recommend strict safety compliance. TBAB has not shown persistent bioaccumulation, but regular monitoring in wastewater runoff prevents surprises. Environmental officials issue guidance for safe disposal into approved chemical waste streams, since undiluted TBAB can stress aquatic systems. With regulators pushing for greener processes, the chemical industry responds by limiting unnecessary TBAB discharge and recycling as much as possible within closed systems.
Future Prospects
Spending time with innovation-minded chemists, it becomes clear TBAB keeps evolving to fit new fields. Startups and multinationals try more sustainable feedstocks and greener reaction pathways, using TBAB as a substitute for older, more toxic phase-transfer agents. Designers of functional materials and nanostructures explore TBAB-based building blocks, eyeing healthcare and wearable tech applications. Battery developers and materials scientists keep searching for electrolytes and dispersants with better temperature stability and conductivity, with TBAB on their radar. As regulations tighten on hazardous waste and energy inefficiency, TBAB’s benign profile may unlock doors shut to harsher chemicals. Demand for custom synthesis of TBAB analogs also picks up as specialty chemicals become more important to biotech, pharmaceuticals, and clean energy. The journey from 20th-century workhorse to 21st-century innovation partner keeps rolling forward, driven by creative application and a push for safer, more efficient science.
Understanding Its Purpose
Tetrabutylammonium bromide, often found on the shelf in chemistry labs, shows up in more reactions than most might expect. From the outside, it just looks like a standard white powder. Dig a little deeper and it tells more than one story. This chemical steps into roles that make tough reactions work smoother and sometimes possible at all.
Breaking Barriers in Chemistry
I remember working with phase-transfer catalysts in a college lab class, where moving a molecule from one place to another in a mixture became a challenge. Here comes tetrabutylammonium bromide. Its structure, with bulky organic tails and a bromide ion, acts like a bridge between water and oil—two worlds that usually don't mix. In experiments where water-based and organic chemicals need to meet and react, this compound guides the show. It pulls ions across boundaries that would otherwise stop reactions cold.
This ability lets chemists skip over some of the toxic or expensive solvents that used to be standard in organic synthesis. Bringing reactions together in mild conditions helps save both money and trouble with hazardous waste. The push for greener chemistry gets a real boost here, backed by studies showing how phase-transfer catalysts support safer and more efficient processes.
Changing the Game Beyond Synthesis
The uses don't stop with school experiments or academic research. Tetrabutylammonium bromide finds work in making pharmaceuticals, cleaning up contaminated water, and even prepping samples for analysis. Drug makers need purity, and this compound helps separate and clean up their products. Wastewater experts use it to trap toxic metals and make them easier to filter out. I’ve seen researchers praise its steady hand in making chemical extractions sharper and more reliable.
Strong Safety Record, Real Risks
No chemical comes without a downside. Common sense tells us to use gloves and goggles around tetrabutylammonium bromide. Most manufacturers provide solid data on handling and exposure. Those working with large amounts should pay attention to airborne dust—breathing it in over time might cause issues. The Environmental Protection Agency has weighed in on various ammonium salts, flagging their persistence in wastewater. Industry needs to look at the whole life cycle, not just the short-term results.
Looking for Practical Solutions
Some chemists experiment with swapping tetrabutylammonium bromide out for biodegradable alternatives. Progress stays slow, since the unique structure delivers results that are tough to match. Others double down on recycling—recovering this catalyst after the reaction and cleaning it up for another round. Equipment in larger installations makes this process less of a headache than it used to be. Universities keep sharing advances so smaller labs can join in.
If science classes and industry keep sharing their findings and watching out for health and safety, the story of tetrabutylammonium bromide won’t get stuck. Safety training and waste management bring peace of mind. Responsible use opens doors for new discoveries, builds stronger medicines, and cleans up the world—step by step, bottle by bottle.
Science on Your Kitchen Table
Tetrabutylammonium bromide often pops up in labs that dive into organic chemistry, especially for phase transfer catalysis. Its name might sound like a mouthful, but those who’ve wrestled with stubborn reactions in a college or industry lab know this salt for how it bridges oil and water—literally. Mix it with water, and you’re tapping into its unique “structure meets function” advantage.
Digging into Solubility
Pour a bit of tetrabutylammonium bromide into a cup of water and watch what happens. The salt dissolves pretty well compared to many organic salts. This quality sets it apart from classic table salt or chalk, where you rub and rub and chunks remain at the bottom. Tetrabutylammonium bromide’s bulky structure with four butyl groups isn’t there for show—it pushes the compound to dissolve better than its cousins with shorter chains or bulkier halides.
Lab data backs this, listing its water solubility at about 45 grams per 100 milliliters at room temperature. Those numbers show that you won’t see much left undissolved unless you dump large amounts in. From a chemist’s viewpoint, this property makes it a handy tool for swapping ions between phases.
Practical Experience Counts
In my own lab work, I reached for tetrabutylammonium bromide while running reactions involving ionic exchanges. Sure, its ionic nature got things going, but if you skip proper mixing or let the solution cool too much, undissolved granules can make measurements unreliable. Having clear insights from years hunched over glassware, I’ve learned a little patience—stir long enough, get the temperature right, and you avoid errors in results or wasted material.
It’s tempting to expect every salt to behave the same when tossed in water—especially those with “ammonium” in their name. Tetrabutylammonium bromide flips that expectation on its head. Its organic cation dances nicely with water molecules, letting users skip the drama of endless heating or aggressive shaking. No one wants a clogged filter or half-dissolved mess in their sample vial.
Why This Matters for Real Life
Good solubility means better control during synthesis, faster reactions, and clearer results. Sloppy dissolving calls for wasteful repeats and more chemical disposal. With environmental discussion on everyone’s lips, making each lab process leaner fits the drive toward green chemistry. You don’t need to dump harsh solvents into the mix or push up temperatures—just weigh, pour, mix, and you’re moving ahead.
Undergraduate labs benefit as well. Complex protocols lose appeal if students guess at what’s fully dissolved. Simple, predictable solubility brings confidence and keeps mistakes low in group projects or final exams.
Room for Better Solutions
Some setbacks pop up if solutions cool down fast. Precipitation ruins measurements. Insulating flasks or sticking with smaller volumes often keeps everything running smooth. People have also explored structural tweaks to the salt for even better behaviors—shorter or branched chains might give more options to meet needs in specialty projects.
Better training helps a lot. If students and new researchers learn how solubility changes with temperature, concentration, and mixing, mistakes shrink in number. Accurate, clear guidelines on working with tetrabutylammonium bromide spread throughout labs boost both safety and productivity.
Shaping the Future with Thoughtful Use
Simple choices in the lab—like picking a highly soluble salt—add up. By relying on well-characterized materials such as tetrabutylammonium bromide and understanding how they interact with water, anyone involved in chemistry, from student to seasoned researcher, puts themselves in a position to work more safely, efficiently, and in harmony with sustainability goals.
Understanding Direct Measurements in Chemistry
Knowing the molecular weight of a compound isn’t just a box to check in high school chemistry. In my own lab work, I’ve witnessed how the accuracy of a simple number like molecular weight can spell the difference between success and wasted resources. Tetrabutylammonium bromide, a quaternary ammonium salt widely used in various organic syntheses, sports a molecular weight of 322.37 g/mol. This number might seem dry on paper, but in a working lab, it’s a baseline. Every time someone prepares a solution or scales up a reaction, that figure comes into play.
Relevance in Everyday Lab Practice
Tetrabutylammonium bromide doesn’t just pop up in the background. It’s found in phase transfer catalysis, analytical chemistry, and sometimes even as a supporting electrolyte. I remember trying to optimize a Suzuki coupling and using this salt to shuttle reactants across phases. Undercutting or over-shooting the amount could mean sluggish reactions or side products. Weighing out the wrong mass based on an incorrect molecular weight led me down that path early in my career. After one messy run, I double-checked my numbers—322.37 grams per mole. Lesson learned, and less waste.
Impacts on Research Quality and Trust
Mistakes in calculations erode trust in research. Publications and grad students both rely on data that can be reproduced. If another group runs experiments based on skewed molecular weights, frustration spreads—just as I’ve heard in private chats with other staff researchers. Academic rigor demands accuracy. Journals care about transparency, and so do funding agencies. The more scrupulously a chemist calculates and reports, the more credibility the study earns.
The E-E-A-T framework promoted by Google—Experience, Expertise, Authoritativeness, and Trustworthiness—gets real traction in science reporting through precision. In the crowded world of chemical research, every detail counts. If a PI or lab technician makes a habit of skipping the details, trust evaporates quickly. Sharing spot-on data on something as straightforward as molecular weight demonstrates respect for readers and peers.
Potential Solutions for Avoiding Errors
One solution has always worked for me: double verification. Whether rushing or painstakingly slow, peer-checks inside the lab catch simple mistakes. Comprehensive digital tools like ChemDraw and internet-based calculators help, but printed references on the bench still have a place. Posting standard molecular weights for widely-used chemicals like tetrabutylammonium bromide right near the balances can nudge accuracy up—especially during long syntheses or on Monday mornings after little sleep.
Constant education in the lab about double-checking units and calculator entries nurtures good habits. It’s humbling to revisit a college-level concept and see how often adults get tripped up by basic math under stress. In group meetings, sharing those near-misses helps foster an open culture where folks can admit mistakes and adjust.
Direct Benefits of Getting the Basics Right
By prioritizing accuracy in something as basic as molecular weight, labs can cut waste, speed up troubleshooting, and hold onto precious funding. The bottom line? Every chemist, whether novice or seasoned, stands to gain from a straight answer: Tetrabutylammonium bromide carries a molecular weight of 322.37 g/mol. The knock-on effect is reliable science, smoother teamwork, and fewer 2 a.m. head-scratchers.
Why Storage Practices Matter
Tetrabutylammonium bromide shows up in many labs. Chemists count on it for its roles in phase transfer, synthesis, and analytical work. Even so, storing this compound isn’t just about putting it on a shelf. Mishandling might lead to wasted material, lowered quality, or safety headaches nobody needs. My own early days in a university lab taught me that proper storage saved not just chemicals but also plenty of time and money. If you want reliable results and a safer workspace, storage decisions can’t be an afterthought.
Keeping It Dry and Sealed
This compound attracts water from the air and clumps up. If you’re not careful, opening a jar too often or leaving it in a humid spot fills it with moisture. After weeks of bad storage, a previously free-flowing powder turns into a sticky, unusable mess. A tightly closed cap and a desiccator make a huge difference. An extra step after using the compound—wiping the rim and making sure the lid seals—keeps out the dampness. Each gram costs money, and careless habits can ruin an entire batch.
Temperature Control Means Stability
I’ve seen plenty of chemicals go off because they were stashed next to ovens, hotplates, or in rooms without climate control. Tetrabutylammonium bromide handles moderate room temperatures, but leaving it in direct sunlight or near warm equipment cuts its shelf life. Light might not break it down overnight. Yet, over months, quality drops. A dark cabinet away from any heat source works better than fancy refrigerators for this one. Temperature swings in a cluttered storeroom create condensation inside bottles, starting a chain reaction that spoils what’s inside. Reliable compounds fuel repeatable science.
Avoiding Cross-Contamination
Using the same spatula across multiple bottles creates a mess before anyone even realizes it. Sodium chloride dust or solvent droplets from another bottle transfer straight to your tetra compound, and purity plunges. In one student project I supervised, just a pinch of contamination ruined ongoing analyses. Always use clean scoops for every sample and don’t let glassware or tools double up on jobs. Label containers loudly and clearly, so nobody mistakes that white-topped bottle for something else. Smart labeling and strict habits simplify cleanup and save budgets across entire semesters.
Safety Must Take Center Stage
A chemical on a bench often looks harmless, but under the wrong environmental conditions, risks pop up. Spilled powders scatter across benches if jars fall or caps go loose. Breathing in dust over the workweek, even if accidental, adds up. Keep tetrabutylammonium bromide away from eating zones and eye level to avoid accidental spills. Post simple storage instructions near shelves, especially if the lab has new hands cycling through every season. Spill kits, gloves, and goggles turn minor mistakes into non-issues.
Encouraging Ongoing Training and Checks
Too many labs take a “set and forget” approach. Their storage corners slowly fill with old, degraded chemicals because nobody reviews them until disaster hits. Building in regular checks, clearing out outdated bottles, and opening discussions about smart storage pay off year after year. If everyone in the lab stands on the same page, from green students to experienced techs, chemicals get treated with the respect they deserve. That foundation supports good science, healthy budgets, and, most importantly, safe people.
Recognizing the Chemical’s Potential Risks
Tetrabutylammonium bromide shows up in a lot of chemical labs, mostly as a phase transfer catalyst or to support organic synthesis. Even so, it deserves some real respect. Many people I’ve worked with skip over the safety data because they assume anything without a skull and crossbones looks harmless. But skin and eye irritation is a real risk with this compound. Accidental spills can leave hands red and irritated for days if you’ve skipped gloves. Even lint from contaminated gloves can leave skin itching where you least expect it. The Safety Data Sheet from Sigma-Aldrich clearly marks out its irritant properties, and the experience matches up. If it gets in your eyes, the burn feels immediate. Flush right away with water.
Practical Protective Gear
Nitrile gloves beat latex here. After several long lab days, the nitrile types always last longer without splitting. Lab coats must go all the way to the wrist and shouldn’t have gaps. Getting compound on your own T-shirt makes for a ruined afternoon. Safety goggles are critical, not just regular eyeglasses. Fogged goggles only get noticed during a spill, and by then it’s too late. I always recommend closed shoes — sandals, even in hot weather, invite some of the nastiest small lab accidents.
Handling and Storage
I’ve watched people scoop from an open bottle right over their work area. A gust from the air vent and powder spreads before you see it coming. Weighing this compound inside a fume hood saves a lot of cleaning and health worries. There’s no sharp odor, but it doesn’t mean the substance is safe to inhale. Dust control becomes more important than folks realize. Store the bottle in a dry, cool spot, away from acids and strong oxidizers. Humidity and sunlight cut down shelf life, and clumping makes precise measurements a pain. Writing the date on containers helps track freshness.
Spill Response Wisdom
Small spills are common. Gently scooping up crystals with a spatula and then wiping down with a damp towel gets most of it. The towel goes straight to hazardous waste, not the trash. Trying to sweep powder with a dry broom, like I’ve seen students do, only spreads it around. For bigger spills, grab the spill kit. There’s no shame in asking for help if you’re not sure how to handle it. It’s safer to delay an experiment for cleanup than gamble with your health.
Waste Disposal and Clean Work Habits
Bottle leftovers and contaminated consumables go into specialized waste labeled as halogenated organics. Do not pour down the drain; some lab mates learned that the hard way when the building manager showed up to ask about clogged pipes and warning alarms. Regularly wiping down benches with water and ethanol keeps residue from building up. A messy workspace lets accidents sneak up.
Knowledge and Team Safety
I’ve found that most safety slip-ups happen when people are rushing or trying to impress. Taking fifteen seconds to check the SDS or to double-check glove fit gives peace of mind far beyond the chemical’s purchase price. Sharing real stories of what goes wrong helps the next person avoid injury. Every safety sign in labs exists because someone learned the lesson the hard way. Tetrabutylammonium bromide won’t bite, but treating it casually is a mistake. Respect goes a long way in keeping hands, eyes, and lungs out of trouble.